Baking soda and acid reactions in baking make things puff and rise. But how could you use this same chemical reaction to blow up a balloon?
Background
The reaction between baking soda and acid has been known for a long time. Its most common use is in baking, where the carbon dioxide gas that is produced makes things puff and rise. The first known instance of using baking soda for this purpose was from a cookbook from 1796!
Key Concepts
- Chemistry
- Conservation of Matter
Materials
- Empty Bottle
- Spoon
- Funnel
- Baking Soda (Sodium Bicarbonate)
- Vinegar (Acetic Acid)
- Balloon
Preparation
- Pour about 4 tablespoons of vinegar into the bottle
- Stretch the end of the balloon over the small end of the funnel, and put a generous scoop of sodium bicarbonate into the funnel so it falls into the balloon
- Remove the funnel, and stretch the end of the balloon over the top of the bottle with vinegar in it, but make sure the sodium bicarbonate stays in the balloon.
- Make a prediction: What will happen to the balloon when you add the bicarbonate soda to the vinegar?
- Carefully tip the balloon upright, so the baking soda falls out of the balloon and into the vinegar.
- Let the reaction happen and observe what happens to the balloon.
- With the help of an adult, repeat the experiment in a measuring cup without the balloon. Carefully pour the measuring cup (without spilling the liquid at the bottom out) over a lit candle and see what happens!
- Be sure to clean up when you are done. The contents of the bottle can be poured down the sink, and the balloon can be thrown away. Wash anything that needs to be washed, and put things away where they belong.
Observation and Results
You should see the baking soda and vinegar mixture start to fizz. This is because the acetic acid and sodium bicarbonate are reacting together to produce sodium acetate, carbon dioxide gas, and water. The carbon dioxide fills up the balloon and inflates it, and the other things stay dissolved in the water.
Try adding a challenge for yourself by creating your tower for a specific purpose. Can you make a tower that holds an egg 6 inches off the table? How about a book? How tall can you make a tower using only unbroken pieces of spaghetti? Keep experimenting with different ways to build.
Safety First and Adult Supervision
- Follow the experiment’s instructions carefully.
- A responsible adult should assist with each experiment.
- While science experiments at home are exciting ways to learn about science hands-on, please note that some may require participants to take extra safety precautions and/or make a mess.
- Adults should handle or assist with potentially harmful materials or sharp objects.
- Adult should review each experiment and determine what the appropriate age is for the student’s participation in each activity before conducting any experiment.
Next Generation Science Standard (NGSS) Supported - Disciplinary Core Ideas
This experiment was selected for Science at Home because it teaches NGSS Disciplinary Core Ideas, which have broad importance within or across multiple science or engineering disciplines.
Learn more about how this experiment is based in NGSS Disciplinary Core Ideas.
Engineering Design (ETS)1: Engineering Design
Grades K-2
- 2-PS1-1. Different kinds of matter exist and many of them can be either solid or liquid depending on temperature. Matter can be described and classified by its observable properties.
Grades 3-5
- 5-PS1-1.
- Matter of any type can be subdivided into particles that are too small to see, but even then, the matter still exists and can be detected by other means.
- A model shows that gases are made from matter particles that are too small to see and are moving freely around in space. This can explain many observations, including the inflation and shape of a balloon and the effects of air on larger particles or objects.
Grades 6-8
- MS-PS1-1.
- Substances are made from different types of atoms, which combine with one another in various ways.
- MS-PS1-4
- Gases and liquids are made of molecules or inert atoms that are moving about relative to each other.
- In a liquid, the molecules are constantly in contact with others.
- In a gas, they are widely spaced except when they happen to collide.
- In a solid, atoms are closely spaced and may vibrate in position but do not change relative locations.
- The changes of state that occur with variations in temperature or pressure can be described and predicted using these models of matter.
Grades 3-5
- 5-PS1-4. When two or more different substances are mixed. A new substance with different properties may be formed.
Grades 6-8
- MS-PS1-2. Substances react chemically in characteristic ways.
- MS-PS1-3. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants.\nMS-PS1-6. Some chemical reactions release energy, others store energy.
Explore Additional Science at Home Videos and Activities
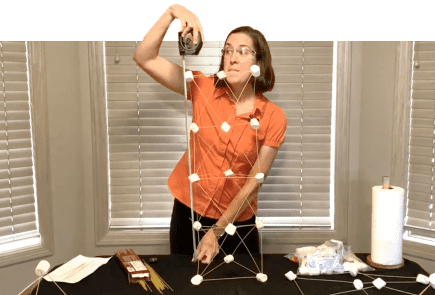
Marshmallow Tower
Have you ever wondered how skyscrapers can be so tall?
Or how people build bridges to span long distances? Explore engineering techniques to build sturdy structures using only marshmallows and uncooked spaghetti.

Elephant Toothpaste
Join Camille Schrier, a scientist who was crowned Miss America 2020, as she shows you how to make a chemical reaction so big it’s fit for elephants!

Rainbow Bubble Snake
Everyone loves bubbles, but have you ever thought about how they form?
In this video, special guest, Kate the Chemist, shows you the science behind bubbles. With just a few simple materials, you’ll learn how to make a bright and colorful bubble snake using your breath, soap, water and a plastic water bottle.

